
By Fernando Aprile García
Head of Applications
Induced pluripotent stem cells (iPSCs) are revolutionizing biomedical research and healthcare by enabling the generation and modification of virtually any cell type to suit various applications. iPSCs are used for disease modeling, drug discovery, and personalized medicine, and the therapeutic potential of iPSCs is vast. Allogeneic cell therapies using iPSCs allow researchers to hurdle perennial barriers like scalability and ethical concerns while enabling the creation of “off-the-shelf” cell therapies for a wide range of diseases. This article will cover the applications of iPSCs with an emphasis on iPSC-derived allogeneic cell therapies in regenerative medicine. The advantages of this technique will also be discussed, as well as the challenges that need to be overcome for widespread clinical use.
Cellular Reprogramming
iPSCs are derived from differentiated cells by adding different transcription factors collectively referred to as Yamanaka factors. These factors are named after Shinya Yamanaka, who pioneered cellular reprogramming in 2006. Once iPSCs are obtained, they can be differentiated into various cell types for diverse research and clinical applications (Fig. 1).
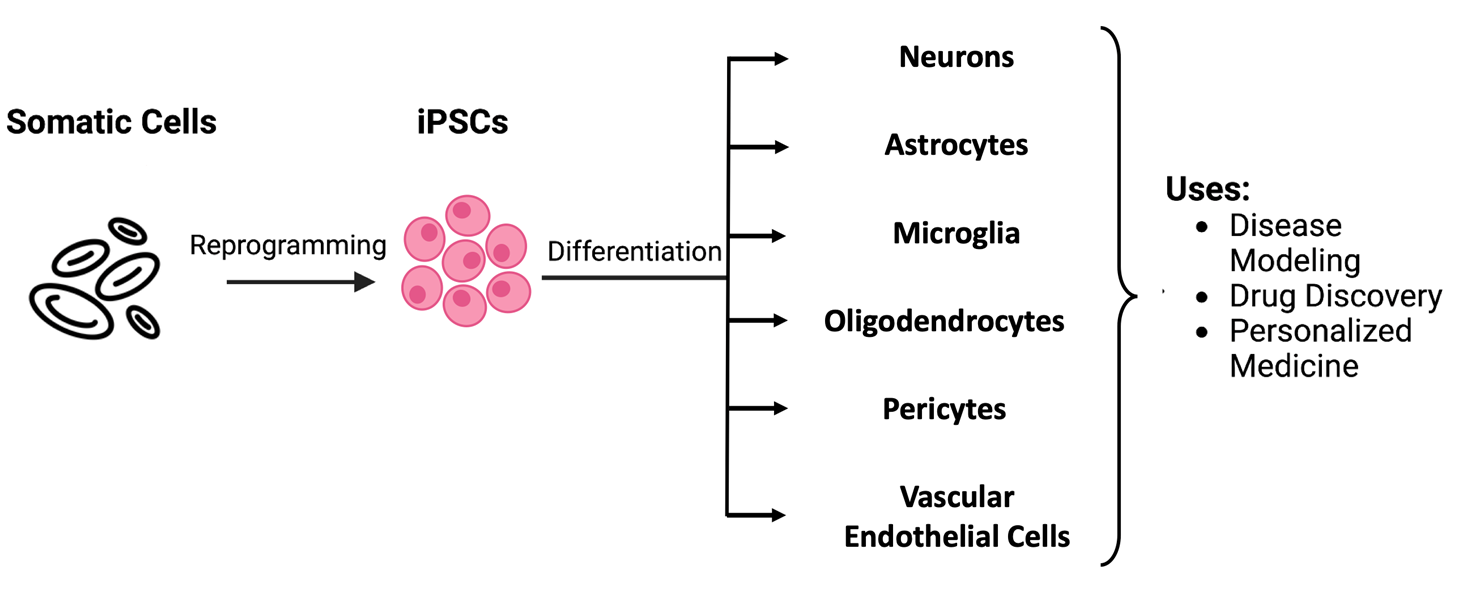
Figure 1. iPCS technology supports various areas of biomedicine by facilitating the production of diverse cell types.
Applications of iPSCs
Disease modeling
Models that more accurately represent a disease state are essential for continued improvements in biomedicine. The pluripotency of iPSCs means they can be used to model a massive variety of diverse diseases. In many cases iPSCs are genetically modified using techniques like CRISPR so scientists can study the effects of mutation on different diseases. For instance, iPSCs can be differentiated into dopaminergic neurons and modified with disease-associated variants to study Alzheimer’s disease. iPSCs can also be differentiated into cardiomyocytes to study cardiovascular disease. In this context, researchers have introduced mutations to ion channel genes to better understand variants that contribute to diseases like Long QT syndrome.
Drug discovery
iPSC and gene editing technology streamline drug discovery by providing more accurate disease models against which potential therapies can be screened. For example, iPSCs can be engineered to carry genetic variants that make cells resistant to certain drugs, providing an excellent model for screening compounds that can overcome these resistance mechanisms7.
Personalized medicine
The therapeutic potential of iPSC-derived allogeneic cell therapies is massive in personalized medicine. Donor cells can be differentiated and modified into virtually any cell type and modified to overcome barriers like immune rejection. This highlights the therapeutic potential of iPSCs, as cells can be tailored to meet a specific patient’s needs. In regenerative medicine this means iPSC-derived allogeneic cell therapies can be used to replace tissues that are damaged or lost in disease, including heart disease (cardiomyocytes) and diabetes (pancreatic β-cells).
Advantages of iPSC-derived Allogeneic Cell Therapies
There are several advantages to using iPSC-derived allogeneic cell therapies.
Universality
iPSCs can be taken from a donor, differentiated, scaled up, and stored to provide a theoretically limitless supply of different cell types that can immediately be incorporated into a patient’s treatment regime. This saves time over autologous methods, in which cells must be extracted from the patient and modified before they can be used.
Scalability and Standardization
iPSC-derived allogeneic cell therapies are more resource- and time-efficient to develop than autologous strategies, which require a separate workflow for each patient. Therefore, allogeneic cell therapies are suitable for mass production, which offers economies of scale and standardized regulatory requirements.
Challenges and Limitations
Immune Rejection
While genetic modifications have significantly decreased the probability of rejection, this remains a risk that potentially limits the therapeutic potential of iPSC-derived allogeneic cell therapies. Discovering more efficient and effective ways to side-step immune rejection is an area of active research.
Tumorigenicity
iPCS therapies carry a risk of tumor formation in patients either due to incomplete differentiation or genomic instability. Cells can be modified to diminish this risk, however.
Cost of Production and Regulatory Alignment
As a relatively new therapeutic area14, iPSC-derived allogeneic cell therapies undergo a rigorous and expensive development and regulatory pathway. Due to the safety concerns mentioned above regulatory bodies require robustly documented proof of cell line monoclonality which can be challenging to provide with manual methods.
CYTENA Case study: Cellistic
Cellistic struggled with generating monoclonal cell lines from iPSCs. Among their issues were an inability to easily prove monoclonality and the sensitivity of iPSCs to shear stress. The UP.SIGHT from CYTENA solved these problems in one go, by providing dual imaging capabilities to prove monoclonality and delicate dispensing to improve the viability and function of iPSCs (Fig. 2). You can read more about Cellistic’s success story here.

Figure 2. The UP.SIGHT from CYTENA has dual imaging technology and delicate single-cell dispensing that provides a >99.99% probability of clonal derivation, making it easy to prove monoclonality to regulators.
Conclusion
iPSC-derived allogeneic cell therapies represent a groundbreaking advancement in regenerative medicine, offering promising solutions for a variety of diseases. Their potential to overcome scalability and standardization concerns makes them a powerful tool for creating “off-the-shelf” treatments. At CYTENA, we’re excited for the next generation of therapies in the pipeline. That’s why we created a cell and gene therapy tracker to keep tabs on the progress of new therapies. Companies producing these therapies must overcome challenges such as tumorigenicity, immune response, and production costs to ensure widespread clinical adoption. Continued research and technological innovation, such as the UP.SIGHT from CYTENA, will be crucial in realizing the full potential of iPSC therapies.
Are you ready to join the revolution in iPSC-derived allogeneic cell therapies? Contact the CYTENA team today to learn more about the UP.SIGHT or book a demo!
References:
- Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Sig Transduct Target Ther. 2024;9(1):112. doi:10.1038/s41392-024-01809-0
- Hui KK, Yamanaka S. iPS cell therapy 2.0: Preparing for next‐generation regenerative medicine. BioEssays. Published online June 25, 2024:2400072. doi:10.1002/bies.202400072
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663-676. doi:10.1016/j.cell.2006.07.024
- Liu C, Oikonomopoulos A, Sayed N, Wu JC. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 2018;145(5):dev156166. doi:10.1242/dev.156166
- Penney J, Ralvenius WT, Tsai LH. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry. 2020;25(1):148-167. doi:10.1038/s41380-019-0468-3
- Funakoshi S, Yoshida Y. Recent progress of iPSC technology in cardiac diseases. Arch Toxicol. 2021;95(12):3633-3650. doi:10.1007/s00204-021-03172-3
- Chehelgerdi M, Behdarvand Dehkordi F, Chehelgerdi M, et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol Cancer. 2023;22(1):189. doi:10.1186/s12943-023-01873-0
- Aboul-Soud MAM, Alzahrani AJ, Mahmoud A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells. 2021;10(9):2319. doi:10.3390/cells10092319
- González BJ, Creusot RJ, Sykes M, Egli D. How Safe Are Universal Pluripotent Stem Cells? Cell Stem Cell. 2020;26(3):307-308. doi:10.1016/j.stem.2020.02.006
- Jha BS, Farnoodian M, Bharti K. Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl Med. 2021;10(2):198-208. doi:10.1002/sctm.20-0242
- Petrus-Reurer S, Romano M, Howlett S, Jones JL, Lombardi G, Saeb-Parsy K. Immunological considerations and challenges for regenerative cellular therapies. Commun Biol. 2021;4(1):798. doi:10.1038/s42003-021-02237-4
- Sato Y, Bando H, Di Piazza M, et al. Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy. 2019;21(11):1095-1111. doi:10.1016/j.jcyt.2019.10.001
- Martin RM, Fowler JL, Cromer MK, et al. Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nat Commun. 2020;11(1):2713. doi:10.1038/s41467-020-16455-7
- McKenna DH, Perlingeiro RCR. Development of allogeneic iPS cell-based therapy: from bench to bedside. EMBO Mol Med. 2023;15(2):e15315. doi:10.15252/emmm.202115315
Optimizing Monoclonal Antibody Production Workflows
Key Steps in Production
- Cell Line Development: Select high-producing cell lines for antibody production.
- Upstream Cell Culture: Grow cells under optimized conditions to maximize yield.
- Harvesting and Purification: Techniques like filtration and chromatography are used to purify antibodies.
- Quality Control: Ensure rigorous testing throughout the process to maintain high-quality standards.
Optimization Strategies
- Automation: Reduces errors and enhances consistency in cell culture and purification processes (Fig. 2) (Holland & Davies, 2020).
- Single-cell Dispensing: Tools like CYTENA’s UP.SIGHT improves clone selection, ensuring high yield and stable antibody production.
- Picking Hits: High-throughput screening and advanced bioinformatics streamline the identification of top-performing clones (Bauer et al., 2023).
- Good Manufacturing Practice (GMP) Readiness: Implement GMP practices and GMP-compliant tools to ensure quality and compliance for scaling up to commercial production.



