Induced pluripotent stem cell (iPSC)-based allogeneic cell therapies are set to transform many fields of healthcare, especially regenerative medicine1. These therapies mean that donor-derived iPSCs can be modified and scaled up for use as “off-the-shelf” treatments for neurodegenerative, cardiovascular, and retinal diseases, to name just a few. Automation is also emerging as a core pillar of modern drug development workflows, facilitating efficiency, reliability, scalability, and higher throughput. This article will discuss the many benefits of automation and how it leads to more efficient iPSC production and accelerates the next generation of allogeneic cell therapies.
Key Technologies in Automation
Automation technologies span the entire cell therapy development workflow, offering enormous benefits at each stage.
Single Cell Dispensing
Obtaining a monoclonal population is a requirement for cell-based therapies. Manual methods for achieving this are time-intensive and often rely on techniques like limiting dilution, which are inherently error-prone. Automation, on the other hand, all but guarantees clonality by integrating single-cell dispensing technology. Microfluidic systems and advanced imaging technologies are central to automation for single-cell dispensing. The UP.SIGHT from CYTENA exemplifies the latest in single-cell dispensing technology and offers researchers >97% single-cell dispensing efficiency.
Monitoring
Automation in iPSC generation includes monitoring clonal growth and confluency after seeding. These capabilities allow scientists to collect large amounts of data concerning clonal performance and viability without time-consuming manual tracking. This, in turn, enables better decision-making and saves countless hours of tedious labor (Fig. 1).
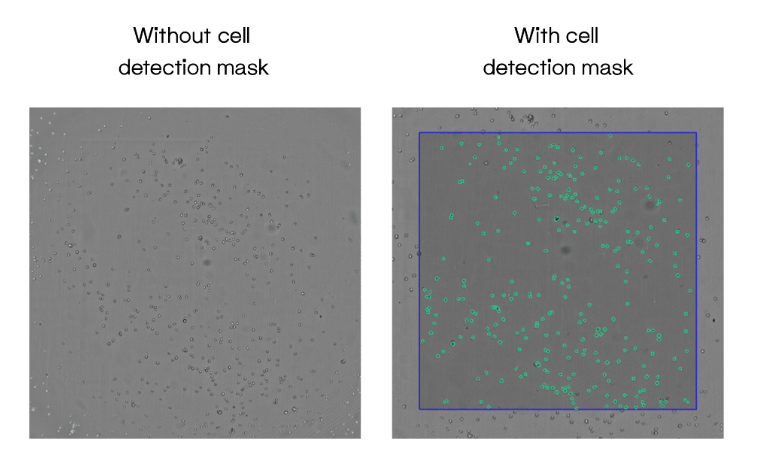
Figure 1: CYTENA’s C.STUDIO software paired with the UP.SIGHT facilitates accurate cell counting and user-friendly reporting.
Cell Culture
Automated cell culture allows for the monitoring of cell concentration and the provision of optimal growing conditions through the replenishing of culture media and the coating of well plates. Robotic handling allows iPSCs to be assayed for efficiency and for faster-growing clones to be selected and scaled up. Automated cell culture workflows facilitate the integration of seeding technologies and bioreactors for efficient iPSC production and expansion, ensuring pluripotent cells with the desired genetic alterations are carried forward. The C.STATION from CYTENA integrates high-end instruments to achieve iPSC cell line development within a single automated platform.
Benefits of Automation in iPSC-based Cell Therapy Generation
Automation enhances virtually every aspect of cell line development workflows, making it an essential consideration for companies looking to pioneer the next breakthrough and get ahead of the competition.
Efficiency
Automation enhances efficiency both in individual tasks and over the longer timelines involved in cell line development. Automated liquid handlers perform tasks more accurately and quickly than human operators and can be programmed to work around the clock to help researchers meet tight deadlines. Researchers can focus on other duties while automated systems perform dispensing tasks, ensuring reproducibility and traceability throughout the cell line development process.
The C.STATION from CYTENA is our complete end-to-end automated cell line development system. It incorporates the UP.SIGHT for delicate single-cell dispensing and our other high-end instrumentation to deliver an efficient and robust cell line development workflow.
Scale
With automation, researchers can work at a scale that best suits their research and is optimized for resource efficiency. Microfluidics systems and precise liquid dispensing mean iPSC-based allogeneic cell therapy workflows can be significantly scaled down to save money on consumables like cell culture medium and multiwell plates. Once the best clones are selected, automation allows these cells to be efficiently scaled up while ensuring optimal growing conditions. iPSCs are prone to losing essential characteristics like pluripotency over time. Efficient scaling means large batches of high-quality iPSCs can be produced quickly before critical traits are lost.
Reduced Contamination
Contamination in iPSC-based allogeneic cell therapy is incredibly costly. Cell lines are at risk of contamination from microbes like mycoplasma, other cell lines and clones, and cells within their clonal population that have lost pluripotency. Each of these scenarios carries unique risks that severely disrupt therapy development pipelines. Automated dispensing and liquid handling significantly reduce the chances of microbial contamination, which is more common with manual methods.
Reliability
Automation in iPSC generation means precise procedures can be repeated within and across laboratories. This reduces the inevitable reproducibility issues that occur with manual methods and multiple human operators. Furthermore, automation provides reliable and accurate dispensing and liquid handling. For example, the UP.SIGHT from CYTENA uses delicate dispensing to ensure that a single iPSC is dispensed without exposing it to shear forces that can compromise growth rates and viability.
Throughput
High throughput is essential for modern therapy development. Automated dispensing means thousands of single clones can be dispensed and validated quickly without manual input. Monitoring many clones produces high quantities of data that automated computerized systems can collect and manage instantly. The UP.SIGHT from CYTENA is paired with the dedicated software C.STUDIO, which collects crucial data on clonality and confluency.
Regulatory Compliance
iPSC-based allogeneic cell therapies require robust documentation to achieve regulatory compliance. This includes precise information about the source of cells, how they were handled, and proof that the population is monoclonal. Automated systems mean researchers can execute reproducible, standardized workflows for iPSC-based cell therapies and provide regulatory bodies with precise protocols that use current good manufacturing practices (cGMP). The UP.SIGHT from CYTENA has dual imaging technology that images cells as they are being dispensed and after they have settled in the well (Fig. 2). It also monitors clones as they expand, providing robust evidence of clonality that helps to streamline therapy development and avoid the common pitfalls of manual workflows.
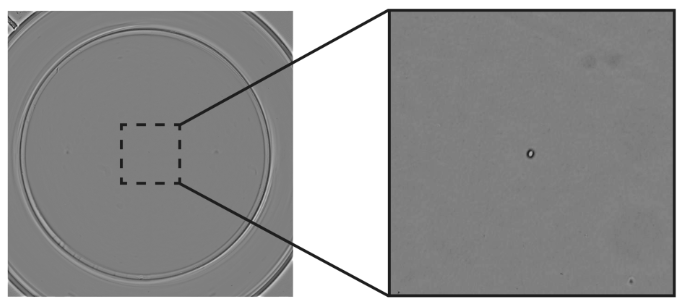
Figure 2: The UP.SIGHT from CYTENA facilitates the detection and monitoring of single clones, helping to ensure regulatory compliance.
Conclusion
Automation is revolutionizing iPSC-based allogeneic cell therapy generation by enhancing efficiency, scalability, and reliability. Automated technologies streamline critical processes such as single-cell dispensing, monitoring, and cell culture, ensuring consistent results and reducing contamination risks. By enabling higher throughput and robust documentation, automation supports regulatory compliance and accelerates the development of next-generation therapies. As a result, automation is pivotal in advancing healthcare, paving the way for more breakthroughs in regenerative medicine.
The UP.SIGHT from CYTENA is a game-changer in cell line development. Schedule a demo today or contact our team for more details on this groundbreaking technology.
References
- Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3. doi:10.3389/fcell.2015.00002
- Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Sig Transduct Target Ther. 2024;9(1):112. doi:10.1038/s41392-024-01809-0
- Schneider G. Automating drug discovery. Nat Rev Drug Discov. 2018;17(2):97-113. doi:10.1038/nrd.2017.232
- Torres-Acosta MA, Lye GJ, Dikicioglu D. Automated liquid-handling operations for robust, resilient, and efficient bio-based laboratory practices. Biochem Eng J. 2022;188(108713). doi:10.1016/j.bej.2022.108713
- Gross A, Schoendube J, Zimmermann S, Steeb M, Zengerle R, Koltay P. Technologies for Single-Cell Isolation. Int J Mol Sci. 2015;16(8):16897-16919. doi:10.3390/ijms160816897
- Doulgkeroglou MN, Di Nubila A, Niessing B, et al. Automation, Monitoring, and Standardization of Cell Product Manufacturing. Front Bioeng Biotechnol. 2020;8:811. doi:10.3389/fbioe.2020.00811
- Schwedhelm I, Zdzieblo D, Appelt-Menzel A, et al. Automated real-time monitoring of human pluripotent stem cell aggregation in stirred tank reactors. Sci Rep. 2019;9(1):12297. doi:10.1038/s41598-019-48814-w
- Tristan CA, Ormanoglu P, Slamecka J, et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells. Stem Cell Reports. 2021;16(12):3076-3092. doi:10.1016/j.stemcr.2021.11.004
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8(571777). doi:10.3389/fbioe.2020.571777
- Madrid M, Lakshmipathy U, Zhang X, et al. Considerations for the development of iPSC-derived cell therapies: a review of key challenges by the JSRM-ISCT iPSC Committee. Cytotherapy. Published online June 2024:S1465324924007308. doi:10.1016/j.jcyt.2024.05.022
- McKenna DH, Perlingeiro RCR. Development of allogeneic iPS cell-based therapy: from bench to bedside. EMBO Mol Med. 2023;15(2):e15315. doi:10.15252/emmm.202115315