Applications of Single-Cell Seeding in Biomedical Research
Regenerative Medicine
iPSCs
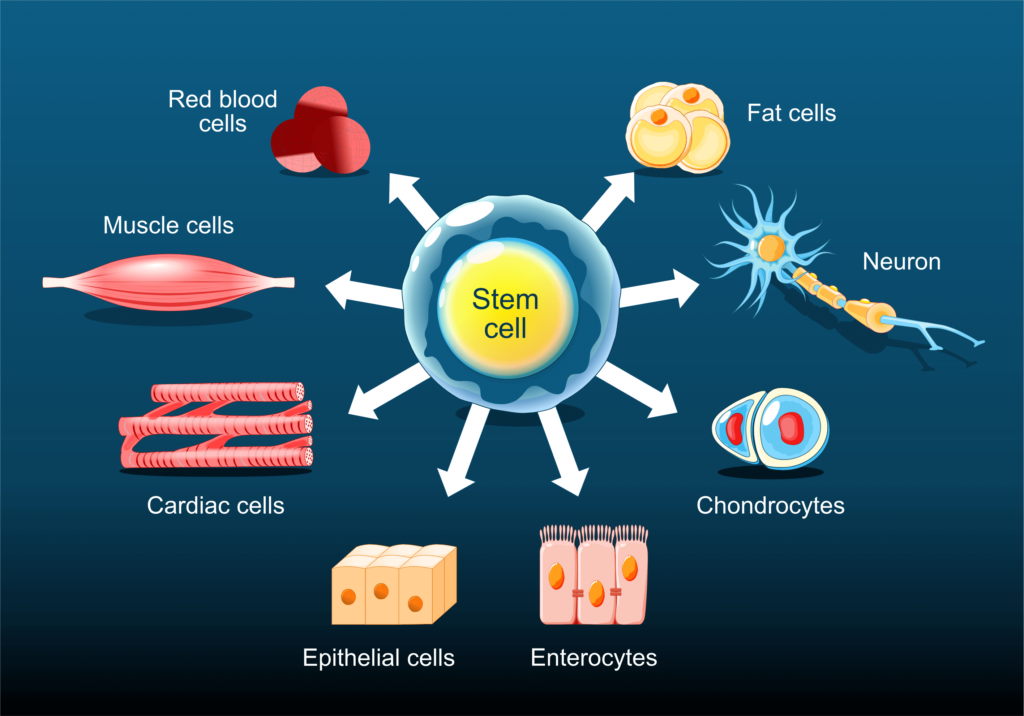
The isolation and manipulation of iPSCs is a cornerstone of regenerative medicine1. These cells can be taken from an individual and differentiated into a cell type that can help replace or grow missing or damaged tissue. Advances in genetic engineering, particularly with the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology, allow donor iPSCs to be altered to overcome issues with rejection. Furthermore, researchers can culture specific stem cells using single-cell dispensing to gain insights into their function and behavior under different conditions.
Accurate single-cell seeding is crucial here for establishing a traceable clonal population that will produce consistent and replicable differentiation states under a given treatment (Fig. 1). Thus, single-cell seeding applications in regenerative medicine impact a wide variety of processes, including:
- Cardiac Regeneration
- Wound Healing
- Spinal Cord Injuries
- Liver Regeneration
- Retinal Regeneration
- Bone and Cartilage Repair
In-vitro Modeling
Oncology: Understanding Tumor Heterogeneity
Cancer Stem Cells
Immune Component
Many tumors possess an immune cell component that helps the cancer resist therapy. Several treatments aim to rearm the immune system to attack the tumor instead of aiding it. Examples include antibody-based therapies like programmed cell-death 1 inhibitors, which prevent cancer cells from “switching off” immune cells6. These therapies require single-cell seeding to provide verifiable, high-yield cell lines that produce antibodies7.
Metastasis
Therapy Development
Drug Screening
Therapy Production
Cells can be used as factories to produce biologic-based therapies or as therapies in their own right. Both cases require accurate single-cell seeding at the beginning of the therapy production process to ensure purity and functionality. Regulatory bodies require robust traceability of cell lines used as or to produce therapies10. Without accurate single-cell seeding, this becomes increasingly difficult, leading to delayed drug discovery timelines and significant loss of resources.
The Challenges of Single-Cell Seeding in Biomedical Research
Biological Challenges
Technical Challenges
The UP.SIGHT All-in-One Single-Cell Dispenser and Imager
This novel technology from CYTENA uses automation and microfluidics11 to ensure fragile cells like iPSCs are exposed to less shear stress and achieve higher recovery rates. The UP.SIGHT’s advanced imaging technology ensures clonality and makes it easy for scientists to select and scale up high performers (Fig. 3). In short, the UP.SIGHT provides a one-stop solution to various biological and technical challenges in single-cell seeding.

Conclusion and Future Directions
References
- Nicholson MW, Ting CY, Chan DZH, et al. Utility of iPSC-Derived Cells for Disease Modeling, Drug Development, and Cell Therapy. Cells. 2022;11(11):1853. doi:10.3390/cells11111853
- Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571-584. doi:10.1038/s41580-020-0259-3
- Hausser J, Alon U. Tumour heterogeneity and the evolutionary trade-offs of cancer. Nat Rev Cancer. 2020;20(4):247-257. doi:10.1038/s41568-020-0241-6
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124-1134. doi:10.1038/nm.4409
- Damen MPF, Van Rheenen J, Scheele CLGJ. Targeting dormant tumor cells to prevent cancer recurrence. The FEBS Journal. 2021;288(21):6286-6303. doi:10.1111/febs.15626
- Sharma P, Goswami S, Raychaudhuri D, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186(8):1652-1669. doi:10.1016/j.cell.2023.03.006
- Parray HA, Shukla S, Samal S, et al. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int Immunopharmacol. 2020;85:106639. doi:10.1016/j.intimp.2020.106639
- Ring A, Nguyen-Sträuli BD, Wicki A, Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2023;23(2):95-111. doi:10.1038/s41568-022-00536-4
- Teng T, Yu M. Establishing Single-Cell Clones from In Vitro-Cultured Circulating Tumor Cells. In: Gužvić M, ed. Single Cell Analysis. Vol 2752. Methods in Molecular Biology. Springer US; 2024:119-126. doi:10.1007/978-1-0716-3621-3_8
- Welch JT, Arden NS. Considering “clonality”: A regulatory perspective on the importance of the clonal derivation of mammalian cell banks in biopharmaceutical development. Biologicals. 2019;62:16-21. doi:10.1016/j.biologicals.2019.09.006
- Liu D, Sun M, Zhang J, et al. Single-cell droplet microfluidics for biomedical applications. Analyst. 2022;147(11):2294-2316. doi:10.1039/D1AN02321G




