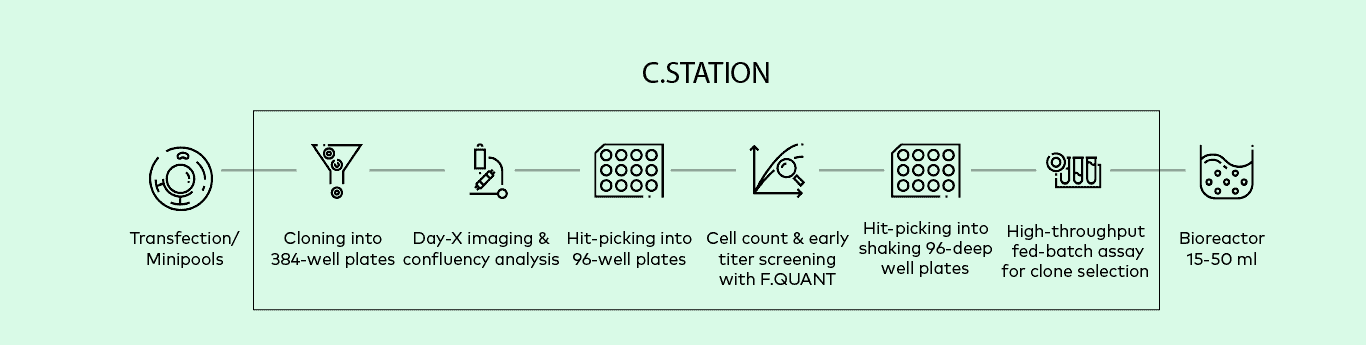
C.STATION™
From single cells to top clones. Fully automated and at scale.
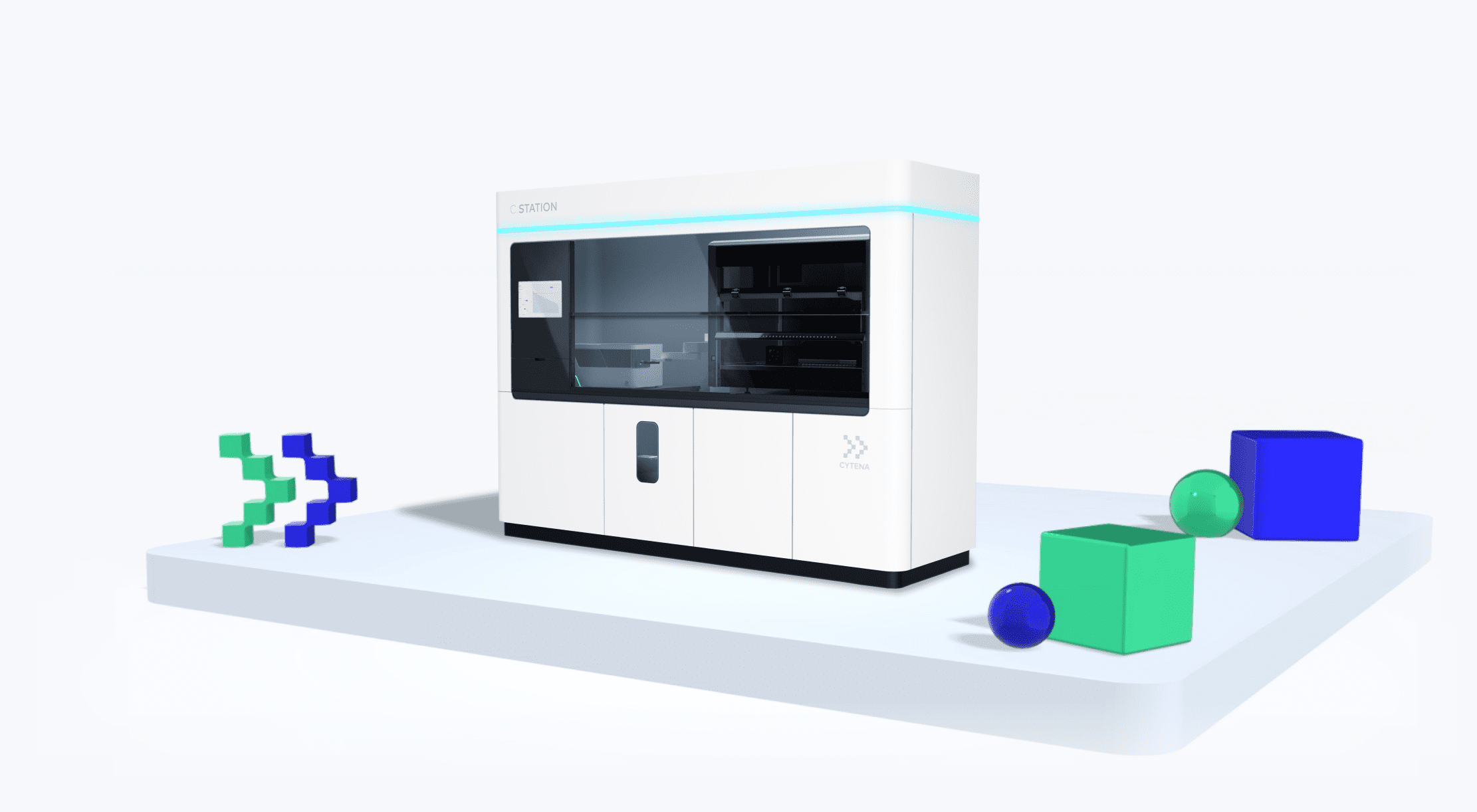
The C.STATION is the first fully automated workstation designed to address all the specific steps in modern stable cell line development (CLD) workflows. This groundbreaking platform integrates proven technologies for single-cell cloning, imaging, and advanced cell culture with cutting-edge automation software and a clone-centric data management system to ensure full traceability and regulatory readiness for the production of modern therapeutics.
The platform offers a scalable solution for developing high-performing genetically modified stable cell lines for monoclonal antibody (mAb) production, viral vector production, and the development of inducedpluripotent stem cell (iPSC)- derived cell lines.
Stop automating single steps. Start automating cell line development.
c-station-video-popup
C.STATION™
Optimize and streamline entire cell line development (CLD) workflows with this automated, intuitively designed workstation. From single-cell cloning of transfected cells to selecting high-producing clones to upscaling, the all-new C.STATION fully automates stable CLD for monoclonal antibody production.
The World’s Only Purpose-built CLD Automation Workstation
From cloning to screening RCB/MCB
candidates - all on one system
Supports GMP & 21 CFR
Part-11 compliance
Clone-centric data
management solution
Biosafety Level 1
& 2 configurations
Full process control with
Green Button Go scheduler
Available for adherent and
suspension cell lines
Join 1,000+ Biopharmaceutical Companies and Academic Institutions



















Key Features & Benefits

The most complete platform for CLD
The C.STATION goes beyond cell cloning and productivity screening. It supports all critical CLD workflow steps to drive research or generate master cell banks (MCBs), including expansion and high-throughput fed-batch culture.

Flexibility and throughput
The C.STATION delivers capacity tailored for modern CLD labs, increasing efficiency and results whether you're running a few campaigns annually or multiple simultaneously. Seed hundreds to thousands of clones without impacting timelines or increasing manual workload.

Adherent and suspension cells
Advanced cell culture operations with both adherent and suspension cells enable broad compatibility with the most important CLD cell lines, including CHO, HEK, and iPSCs.

Regulator-friendly
Produce high-quality cell lines and automated clonality reports that meet regulatory requirements for FDA and EMA applications, including IND and BLA submissions.

Single point of contact
With CYTENA, your procurement team works with a single vendor. Enjoy seamless support from one team, covering the entire workstation.

Predictable timelines and outcomes
The preconfigured workstation deploys in months—not years— allowing you to start quickly with a library of preconfigured methods optimized for modern CLD.
Shorten CLD timelines
Advanced technologies like early titer screening and small-scale, high-throughput fed-batch culture enable early selection of high-performing clones, saving time, reagents, and costly labware.

Complete data management solution
Ensure full traceability, eliminate human error, and manage large data volumes with the integrated clone-centric database and dedicated C.STUDIO and C.SERVICES software.

Made for CLD scientists
Lab automation for complex cell culture workflows has never been more accessible. The C.STATION is designed with the end user in mind, featuring an intuitive interface that eliminates the need for expertise in automation or programming.
Product Details
Achive excellence with the ultimate automated CLD platform
What is inside?
The C.STATION integrates all its components in a compact
workstation in either BSL-1 or BSL-2 configuration.

cell culture operations
Industrial CLD workflows require consistent results and timelines, which can be hard to achieve with manual workflows. The C.STATION enables the automation of even the most advanced cell culture operations required in CLD, such as harvesting, splitting, and maintenance of suspension and adherent cells over many weeks.
single-cell seeding
The C.STATION features CYTENA’s proven single-cell dispensing technology, trusted by hundreds of organizations in biologics development. It rapidly dispenses single cells into 96- and 384-well plate formats, delivering best-in-class single-cell cloning efficiency. Designed with gentle technology, it ensures compatibility with even the most delicate cell types, such as HEK cells and iPSCs. Combined with CYTENA’s proprietary z-stack imaging or bottom-well imaging, the C.STATION provides gold-standard, image-based documentation of clonality, fully compliant with IND/BLA submission requirements.
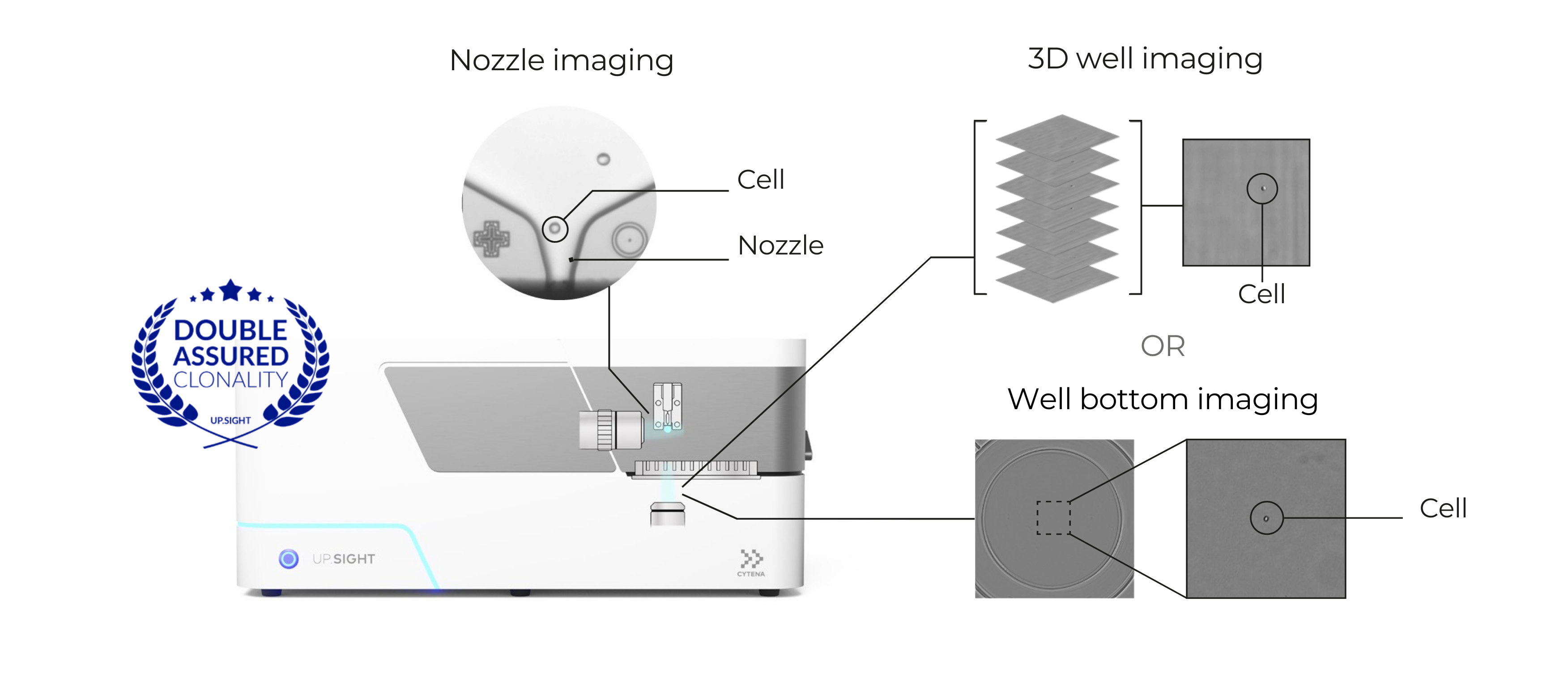
clone screening
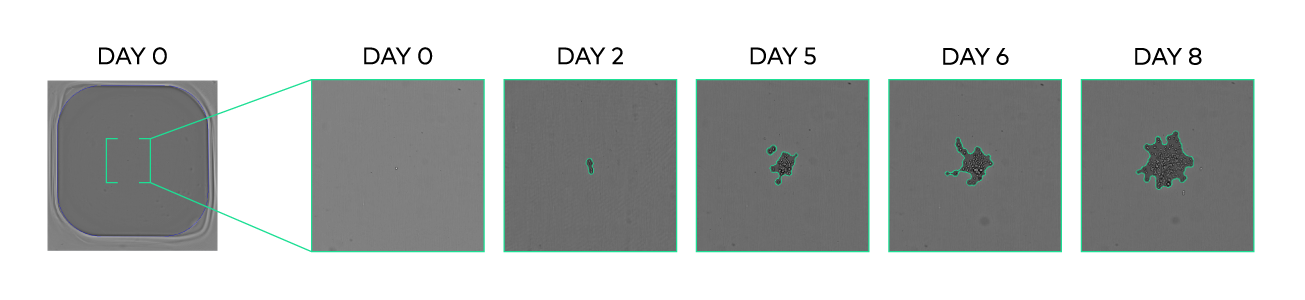
The integration of advanced imaging technology enables day 0 bottom-well imaging, confluency tracking, cell counting, and viability measurements, ensuring fast and accurate clone screening. This seamless integration allows you to select the optimal time point for automated hit picking and plate transfer, based on confluency, cell count, productivity, or other clone screening assay results.
The C.STATION is available in BSL-1 and BSL-2 configurations to match the requirements of diverse laboratories and ensure all the steps of your CLD workflow are performed safely in a controlled and sterile environment.

selection for streamlined CLD
C.STUDIO software’s clone-centricity makes it simple to keep track of all data collected for each individual clone from the single-cell stage to larger well plate formats or suspension culture in deep well plates. The software supports the identification of the best-performing clones at each step and gives you complete freedom to select the optimal time for clone validation or scaling.
integrated or external analytics
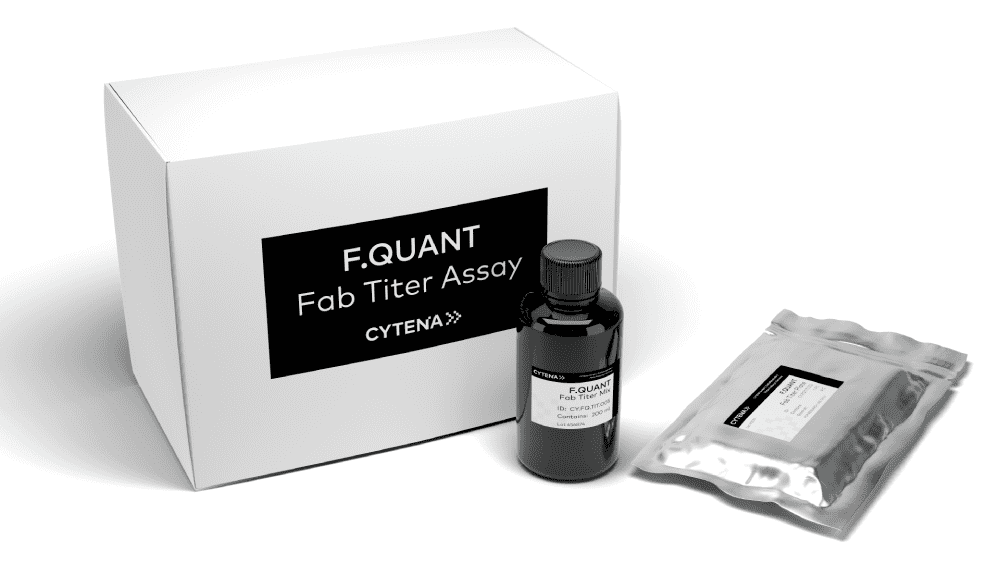
The C.STATION seamlessly integrates technologies for precise measurement of mAb titers, enabling researchers to identify and select high-performing mAb-producing clones. Flexible plate formats and robust data export/import capabilities simplify integration with application-specific analytics, including ddPCR, NGS, and ELISA, for advanced clone characterization and selection.

IND/IMPD submissions
The C.STUDIO software compiles your clones’ picture during single-cell dispensing, z-stack, and bottom-well imaging in a regulator-ready clonality report to support robust proof of monoclonality. The C.STUDIO is compatible with 21 CFR Part-11, ensuring your data integrity, from collection to reporting.

with simple, intuitive software
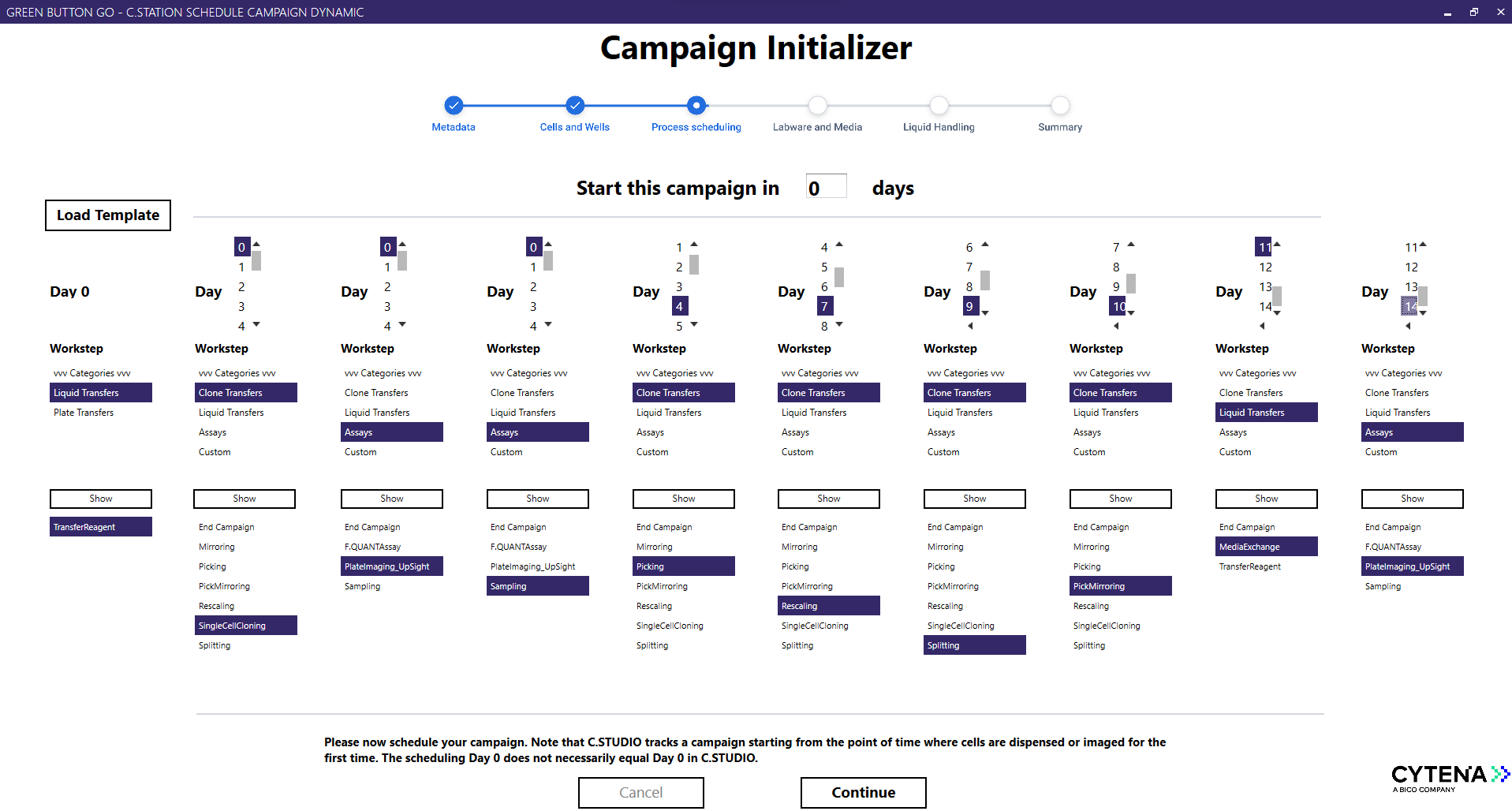
The Campaign Initializer simplifies the setup and management of CLD campaigns, making complex lab automation accessible to scientists focused on achieving timely and successful outcomes. With its intuitive interface, users can leverage a pre-configured library of methods to seamlessly replicate workflows on the C.STATION. For advanced users, the platform offers powerful customization through Green Button Go scheduling software, delivering unmatched flexibility. Complementing this C.STUDIO and C.SERVICES provide a complete solution for clone-centric data visualization and management, rounding out the suite to streamline every step of CLD workflows.
Broad support for
essential cell lines
The C.STATION includes protocols optimized for essential cell lines commonly used in modern therapeutic production, such as CHO, HEK293, Sf9, and iPSCs.

Product
Applications
Automate complex
CLD workflows with
confidence and quality
from end to end

Therapeutic monoclonal antibodies
The C.STATION provides you with the gold-standard solution for mAb-producing CLD. The workflow starts with double clonality assurance and single-cell seeding with the UP.SIGHT within the C.STATION’s BSL-1 environment for suspension cell culture. The C.STATION’s flexible capacity lets you seed thousands of clones, and run multiple campaigns in parallel, making it the ideal solution for both medium to large Biopharma or CDMO companies. C.STUDIO software allows you to monitor each one for confluency and robust growth. The built-in F.QUANT titer assay technology enables seamless quantification of Fc fusion protein or Fab fragment titers, allowing you to select the highest-performing clones early in the process. From here, you can scale up the best clones with the option for high-throughput fed-batch cultivation and screening. In just thirty days, you can generate robust clonal cell lines for mAb production and receive an automated clonality report for immediate engagement with regulatory processes.
Safe and scalable screening of viral vector-producing clones
The C.STATION provides a robust and flexible BSL-2 platform for generating monoclonal cell lines for viral vector production. The double-HEPA-filtered exhaust ensures safety and regulatory compliance and allows suspension culture of cells such as HEK293 and Sf9. The platform enables the efficient generation of assay plates for viral particle quantification and characterization and enables clone selection based on titer and cell count. With the C.STATION, you can run multiple projects simultaneously, dramatically increasing productivity while guaranteeing quality and regulatory compliance.
iPSC-derived cell lines in GMP environment
The C.STATION configuration for adherent cells is engineered to automate the intricate workflows of iPSC culture maintenance, significantly reducing manual interventions that are both time-consuming and resource-intensive. Its gentle, automated single-cell dispensing is tailored for shear stress-sensitive iPSCs, ensuring high viability and robust outgrowth. This GMP-ready BSL-2 adherent cell line workcell minimizes handling while providing precise imaging and comprehensive process documentation to meet stringent regulatory requirements, including clonal assurance and 21 CFR Part-11 compliance. By streamlining the entire workflow—from single-cell cloning to scaling up optimal clones in a matter of weeks—the C.STATION accelerates iPSC development, facilitating the efficient production of iPSC-derived MCBs essential for advancing allogeneic cell therapies and personalized medicine.
End-to-end automated cell line development workflows

Cell therapy
Featured Workflow
With the C.STATION, you will be able to rapidly seed thousands of single-cell clones within
GMP-ready, BSL-1 and -2 environments. Clones are monitored for growth from day 0 and can
be selected for growth and Fc fusion protein or Fab fragment titer then scaled up.
your research with our products and solutions
No Posts Found.
Product Datasheet
| C.STATION Suspension BSL1 | C.STATION Suspension BSL2 | C.STATION Adherent BSL2 | |
| Biosafety Standard | Class I according to NSF/ANSI 49 A2 respect. BS EN 12469:2000 (product protection) HEPA H14 filtered intake | Class II according to NSF/ANSI 49 A2 respect. BS EN 12469:2000 (product and user protection) HEPA H14 filtered intake and exhaust 70/30 recirculation principle with 30% exhausted air | Class II according to NSF/ANSI 49 A2 respect. BS EN 12469:2000 (product and user protection) HEPA H14 filtered intake and exhaust 70/30 recirculation principle with 30% exhausted air |
| Plate Compatibility | ANSI SLAS1-2004 (R2012) without FB module: up to 6-Well with FB module: up to deep-well | ANSI SLAS1-2004 (R2012) without FB module: up to 6-Well with FB module: up to deep-well | ANSI SLAS1-2004 (R2012) up to 6-Well |
| Fed-Batch Option | yes | yes | no |
| Plate Capacity | without FB module: up to 60 plates with FB module: up to 44 plates | without FB module: up to 60 plates with FB module: up to 44 plates | up to 60 plates |
| Cell Sorting | Cell Morphology and Fluorescence on CYTENA UP.SIGHT | Cell Morphology and Fluorescence on CYTENA UP.SIGHT | Cell Morphology and Fluorescence on CYTENA UP.SIGHT |
| Plate Washer | no | no | CYTENA C.WASH |
| Liquid Handling | Hamilton STARlet M | Hamilton STARlet M | Hamilton STARlet M with optional 96-Well head (MPH) |
| Liquid Handling Pipette Tips | 50/300/1000 µL filtered or unfiltered, capacitive tips *unfiltered 50/300 µL tips stackable for 4x capacity on deck | 50/300/1000 µL filtered or unfiltered, capacitive tips *unfiltered 50/300 µL tips stackable for 4x capacity on deck | 50/300/1000 µL filtered or unfiltered, capacitive tips *unfiltered 50/300 µL tips stackable for 4x capacity on deck |
| Liquid Handling Specifications | Minimum/maximum aspirate and dispense volume 50 µL tip 300 µL tip 1000 µL tip | 1-1000 µL depending on tip type @1 µL: 4.0% precision, 5.0% trueness @50 µL: 0.75% precision, 2.0% trueness @200 µL: 0.75% precision, 1.0% trueness @1000 µL: 0.75% precision, 1.0% trueness | 1-1000 µL depending on tip type @1 µL: 4.0% precision, 5.0% trueness @50 µL: 0.75% precision, 2.0% trueness @200 µL: 0.75% precision, 1.0% trueness @1000 µL: 0.75% precision, 1.0% trueness |
| Liquid Handling Troughput | Fill one 96-well microtiter plate with 100 µL samples (new tips for each sample): 320 s Aliquot 100 µL to each well of a 96-well plate, liquid level detection on aspirate: 35 s | Fill one 96-well microtiter plate with 100 µL samples (new tips for each sample): 320 s Aliquot 100 µL to each well of a 96-well plate, liquid level detection on aspirate: 35 s | Fill one 96-well microtiter plate with 100 µL samples (new tips for each sample): 320 s Aliquot 100 µL to each well of a 96-well plate, liquid level detection on aspirate: 35 s |
| Incubation Temperature | RT+5 °C to 37 °C optional cooling: 4°C to 50 °C | RT+5 °C to 37 °C optional cooling: 4°C to 50 °C | RT+5 °C to 37 °C optional cooling: 4°C to 50 °C |
| Incubation CO₂ | 0-20 Vol% CO₂ | 0-20 Vol% CO₂ | 0-20 Vol% CO₂ |
| Incubation Humidity | without FB module: < 95% with FB module: < 80% | without FB module: < 95% with FB module: < 80% | < 95% |
| Plate Shaking for Assays | 200-3000 rpm, constant 2 mm diameter | 200-3000 rpm, constant 2 mm diameter | 200-3000 rpm, constant 2 mm diameter |
| Software | Green Button Go Scheduler, C.STUDIO (Analysis) on Windows 11 | Green Button Go Scheduler, C.STUDIO (Analysis) on Windows 11 | Green Button Go Scheduler, C.STUDIO (Analysis) on Windows 11 |
| Computation | Custom Rack PC | Custom Rack PC | Custom Rack PC |
| Dimensions (W x D x H) | 3500 x 1200 x 2400 mm 137.80 x 47.25 x 94.5 in | 4200x1550x2440 mm 165.50 x 61.10 x 96.10 in | 4200x1550x2440 mm 165.50 x 61.10 x 96.10 in |
| Footprint Service Mode (W x D) | 3500 x 2540 mm 137.80 x 100.00 in | 4650 x 2240 mm 183.10 x 88.25 in | 4650 x 2240 mm 183.10 x 88.25 in |
| Weight | 1500 kg 3310 lbs | 2000 kg 4410 lbs | 2000 kg 4410 lbs |
| Area load | < 400 kg/m² < 85 lbs/ft² | < 500 kg/m² < 105 lbs/ft² | < 500 kg/m² < 105 lbs/ft² |
| Power Supply | 400 VAC, 32 A via IEC 60309 6h (3L+N+PE) option: 3x individually fused line: 230 VAC, 16 A | 400 VAC, 32 A via IEC 60309 6h (3L+N+PE) option: 3x individually fused line: 230 VAC, 16 A | 400 VAC, 32 A via IEC 60309 6h (3L+N+PE) option: 3x individually fused line: 230 VAC, 16 A |
| Ambient Conditions | +15 °C to + 25 °C non-condensing air (30% – 80% rH) | +15 °C to + 25 °C non-condensing air (30% – 80% rH) | +15 °C to + 25 °C non-condensing air (30% – 80% rH) |
We understand that your business has unique needs, To learn more about the C.STATION pricing and a quote tailored to your CLD ambitions, fill out the quote request form. We would be happy to help you find the best plan for your needs and budget and answer any questions you may have. Thank you!
Featured Resources
Donec scelerisque scelerisque neque, non sagittis ligula malesuada at.