Cell Line Development: Comprehensive Insights and Advances
Cell line development is a cornerstone of several modern biomedical applications. It provides essential models for studying diseases and tools for developing the latest therapeutic products1. Researchers leverage the unique benefits of mammalian cell lines and innovations in gene editing, such as clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 and prime editing, to continuously enhance cell line development processes2. Advancements in automation technology are maximizing efficiency in cell line development by enabling higher throughput, reducing contamination risks, and simplifying the selection of high-performing clones3. These developments are transforming cell line development, making it more reliable and effective than ever. This article covers applications, innovations, and challenges in cell line development and introduces CYTENA’s instruments as future-proof solutions for therapy development.
Mammalian Cell Line Development
Mammalian cell line development is essential for both translational and basic biomedical research. It offers cost-effective and manageable models for various diseases and tools for the production of cutting-edge therapeutics1,4.
Applications
Virtually all fields in biomedicine benefit from mammalian cell line development, including recombinant protein production, vaccine production, cell and gene therapies, drug discovery and development, and fundamental research.
Drug Discovery
Mammalian cell lines provide a reproducible system for monitoring cellular responses to drugs and are crucial for high-throughput screening of drug candidates, biomarker discovery, and personalized medicine applications5,6.
Therapy Production
Mammalian cell line development enables the production of complex molecules and antibodies, which are essential biological therapies for a growing list of indications. Technologies like the UP.SIGHT single-cell dispenser and imager ensure high clonal derivation in cell line development, significantly de-risking therapy production pipelines (Fig. 1).

Figure 1. The UP.SIGHT uses microfluidics to ensure gentle single-cell seeding with a clonal recovery rate of up to 80%.
Research
Mammalian cell lines provide stable models for studying various diseases. Gene editing techniques allow researchers to gain deeper insights into disease mechanisms by facilitating gene knockout experiments. In vivo studies using mammalian cell lines often serve as pre-clinical justification for human trials7.
Challenges
Despite the utility of mammalian cell line development, many challenges slow research progress. Overcoming these challenges with advanced easy-to-use instrumentation gives researchers an advantage over the competition. CYTENA provides the automated C.STATION platform, an end-to-end solution that solves multiple perennial problems simultaneously.
Contamination
Mammalian cell lines are prone to contamination from microbes and other cell lines8. Manual liquid handling increases this risk. Automated liquid handlers like CYTENA’s UP.SIGHT reduce contamination risk and ensure monoclonality with >97% single-cell dispensing efficiency.
Yield Issues
Multiple factors contribute to issues with protein production. Suboptimal cell culture conditions reduce cell productivity and issues with protein folding significantly impact the functionality of the final product. To improve folding, researchers can optimize the protein-coding sequence and co-express chaperone proteins that facilitate proper folding. Researchers can also tailor cell culture conditions, such as temperature, pH, and glucose concentration, to suit the cell line they are using.
Selecting the best clone for scale-up is crucial. CYTENA’s F.QUANT titer assay facilitates optimal clone selection by allowing researchers to easily measure the production of their target protein.
Instability
Cell line instability affects therapy manufacturing by diminishing cellular productivity9. Maintaining stable growing conditions and gentle handling are essential for preventing instability and phenotypic shift. Fully automated cell line development workflows like those achieved through the C.STATION help maintain consistency throughout the process and reduce instability.
Storage
Growing and scaling up multiple cell lines simultaneously can put strain on laboratory incubators and storage space. Automated single-cell dispensing and microfluidics allow workflows to be scaled down, significantly minimizing storage needs. The UP.SIGHT allows researchers to grow clones in 96- and 386-well formats, optimizing both space and resource allocation.
Check out our dedicated article on mammalian cell line development for further insights and solutions to common challenges.
Gene Editing in Cell Line Development
Advances in gene editing have transformed how we approach research, drug discovery and therapy production. New techniques like CRISPR-Cas9 and prime editing are setting new standards for efficiency and accuracy in cell line engineering, paving the way for innovative treatments and research breakthroughs.
Applications
Gene editing can be used to enhance target protein production, stability, and proliferation of cell lines used for therapy production2. It also enables the creation of universal donor cells and off-the-shelf CAR-T cells for cancer therapy (Fig. 2)10. Furthermore, gene editing facilitates the reaction of disease models and enables studies of gene function.
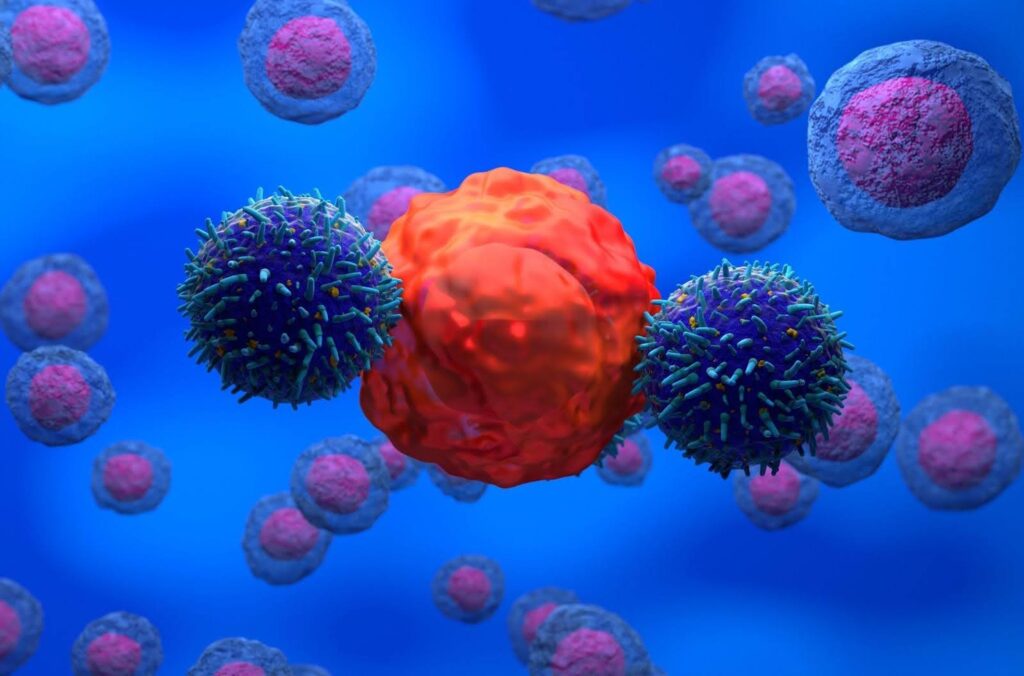
Figure 2. CAR-T cell therapy involves genetically modifying a patient’s immune cells to recognize and attack cancer cells.
Methods for Gene Editing
Novel methods for gene editing are continuously emerging leading to the expansion of applications within cell line development workflows.
CRISPR-Cas9
CRISPR-Cas9 is a groundbreaking gene editing technique that uses guide RNA to direct the Cas9 nuclease to specific genome sequences, causing double-strand breaks for targeted genetic modifications. CRISPR is used to enhance Chinese hamster ovary (CHO) and hybridoma cells for biological therapy production, improving protein yield and cell resilience2.
Prime Editing
Prime editing uses prime editing guide RNA to direct a fusion protein to specific genomic sites, where it integrates precise DNA sequences via a single-strand nick11. Though newer and less extensively used, prime editing holds significant promise for optimizing cell line development by enabling precise genetic modifications.
Read our dedicated article on gene editing for deeper insights and information on other methods of gene editing such as base editing.
Maximizing Efficiency in Cell Line Development
Cell line development is crucial for producing modern therapies, and new automation and genetic engineering technologies are enhancing efficiency and precision across development workflows. New cell line development technologies enable companies to bring novel therapeutics to market faster and more efficiently, resulting in significant cost savings and a n edge over the competition.
Automation
Automation accelerates biomedicine by streamlining cell line development with robotics, AI, and integration of advanced fluid-handling technologies. It enhances efficiency, reduces risks, and frees scientists from repetitive tasks3. Automation also enables high throughput, allowing efficient seeding, screening, and selection of top-performing clones, thus saving resources and time in scaling up production.
Quality Control
Verifying single-cell seeding is crucial for regulatory compliance and therapy development success12. The UP.SIGHT from CYTENA images cells during and after dispensing, providing robust evidence of monoclonality with >99.99% clonal derivation and up to 80% clonal recovery. This instrument allows researchers to expedite their workflows while enhancing the quality of their work.
For more information on optimizing cell line development, including tips on scaling-up and screening, see our dedicated article on maximizing efficiency in cell line development.
Conclusion
Cell line development remains pivotal in advancing biomedical research and therapeutic production. By integrating gene editing and automation innovations, scientists can create more reliable and efficient cell lines in less time. By addressing challenges such as contamination, yield optimization, and cell line stability through cutting-edge technologies like CYTENA’s UP.SIGHT and C.STUDIO, researchers can now achieve higher efficiency and accuracy in cell line development. As these technologies evolve, they promise further to enhance the reliability and effectiveness of cell line development, paving the way for future breakthroughs in biomedicine. CYTENA stands at the forefront of cell line development technologies, helping researchers overcome challenges with advanced, yet simple, solutions.
Visit our website to learn more about our comprehensive suite of instruments that offer end-to-end solutions for cell line development. Ready to see the power of the UP.SIGHT for yourself? A comprehensive demo is just one click away.
References
- O’Flaherty R, Bergin A, Flampouri E, et al. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnology Advances. 2020;43:107552. doi:10.1016/j.biotechadv.2020.107552
- Shen CC, Sung LY, Lin SY, Lin MW, Hu YC. Enhancing Protein Production Yield from Chinese Hamster Ovary Cells by CRISPR Interference. ACS Synth Biol. 2017;6(8):1509-1519. doi:10.1021/acssynbio.7b00020
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8:571777. doi:10.3389/fbioe.2020.571777
- Falkenburger BH, Saridaki T, Dinter E. Cellular models for Parkinson’s disease. J Neurochem. 2016;139 Suppl 1:121-130. doi:10.1111/jnc.13618
- Potekhina ES, Bass DY, Kelmanson IV, et al. Drug Screening with Genetically Encoded Fluorescent Sensors: Today and Tomorrow. Int J Mol Sci. 2020;22(1):148. doi:10.3390/ijms22010148
- Wei F, Wang S, Gou X. A review for cell-based screening methods in drug discovery. Biophys Rep. 2021;7(6):504-516. doi:10.52601/bpr.2021.210042
- Sajjad H, Imtiaz S, Noor T, Siddiqui YH, Sajjad A, Zia M. Cancer models in preclinical research: A chronicle review of advancement in effective cancer research. Animal Model Exp Med. 2021;4(2):87-103. doi:10.1002/ame2.12165
- Weiskirchen S, Schröder SK, Buhl EM, Weiskirchen R. A Beginner’s Guide to Cell Culture: Practical Advice for Preventing Needless Problems. Cells. 2023;12(5):682. doi:10.3390/cells12050682
- Wurm F, Wurm M. Cloning of CHO Cells, Productivity and Genetic Stability—A Discussion. Processes. 2017;5(2):20. doi:10.3390/pr5020020
- Poirot L, Philip B, Schiffer-Mannioui C, et al. Multiplex Genome-Edited T-cell Manufacturing Platform for “Off-the-Shelf” Adoptive T-cell Immunotherapies. Cancer Res. 2015;75(18):3853-3864. doi:10.1158/0008-5472.CAN-14-3321
- Zhao Z, Shang P, Mohanraju P, Geijsen N. Prime editing: advances and therapeutic applications. Trends in Biotechnology. 2023;41(8):1000-1012. doi:10.1016/j.tibtech.2023.03.004
- Welch JT, Arden NS. Considering “clonality”: A regulatory perspective on the importance of the clonal derivation of mammalian cell banks in biopharmaceutical development. Biologicals. 2019;62:16-21. doi:10.1016/j.biologicals.2019.09.006